Direct oral anticoagulants are a relatively new collection of drugs which directly inhibit a single factor in the haemostatic pathway. DOACs can be split into two branches: direct Xa inhibitors including Rivaroxaban, Apixaban, and Edoxaban, and direct IIa (thrombin) inhibitors including Dabigatran. These drugs are fast acting and have a very predictable dose-response relationship and therefore do not usually require monitoring. For this reason, clinical trials for these drugs did not include laboratory testing and consequently we are now discovering, within the Haemostasis laboratory, the effects of these drugs on our specialist assays.
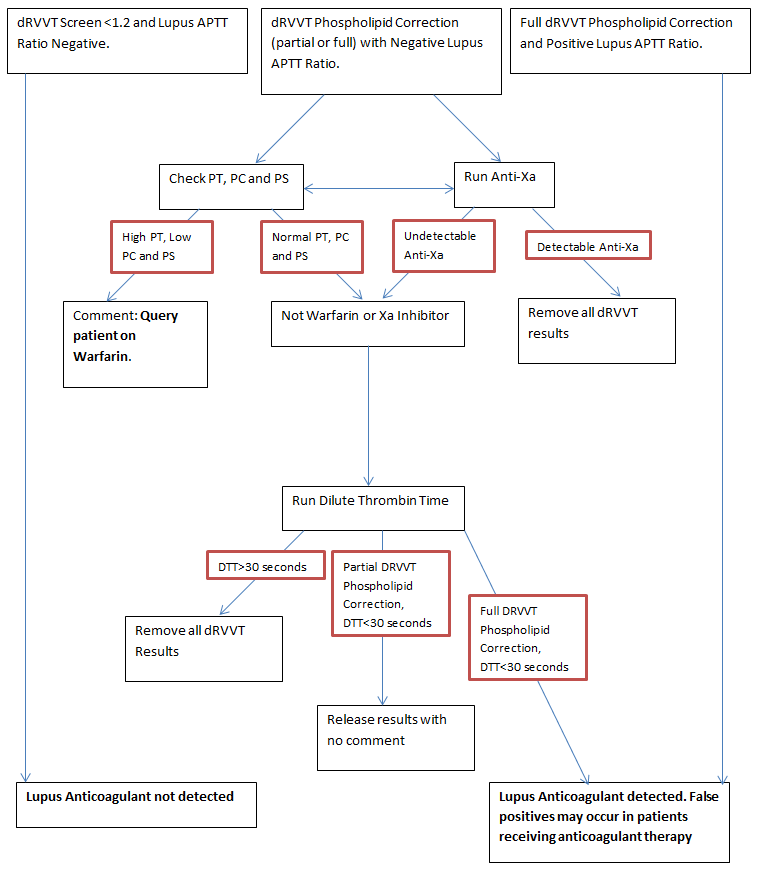
One assay particularly affected by DOACs are dilute Russell’s viper venom time (dRVVT) assays for our Lupus anticoagulant testing. Russell’s viper venom, in both dRVVT screen and confirm reagents, directly activates Factor X to Factor Xa in the presence of calcium. This in turn activates Factor II to Factor IIa and Fibrinogen to Fibrin. The clotting time is measured and subsequently converted into a ratio. The presence of either a direct Xa or IIa inhibitor will falsely prolong the dRVVT screen and confirm. However, the extent of this prolongation is different for both the dRVVT-screen and confirm depending on the type of DOAC present in the plasma. For example, Rivaroxaban has a much greater effect on the dRVVT screen reagent compared to the dRVVT- confirm reagent, which results in an complete phospholipid correction of >22% as seen in the table below. Apixaban and Edoxaban prolong the dRVVT- screen to a lesser extent compared to Rivaroxaban and their dRVVT-confirm result is more prolonged than dRVVT-screen results, producing a negative phospholipid correction. Dabigatran has a similar effect to Rivaroxaban with some correction seen between the dRVVT-screen and confirm assays but not enough to produce a falsely positive correction, in this way it is similar to Warfarin.
In all cases, you will note that the Lupus APTT ratio is negative. Guidelines state that only one of the two assays used for lupus anticoagulant testing needs to be positive to diagnose a patient with a lupus anticoagulant. This is particularly problematic for patients being tested whilst still on Rivaroxaban therefore, if we see these patterns of results we reflex to initially run an anti-Xa assay. If anti-Xa activity is found, the dRVVT-screen, confirm and phospholipid corrections are not reported. However, lack of anti-Xa activity does not rule out the presence of Dabigatran so a dilute thrombin time can also be performed and if found to be prolonged, dRVVT-screen, confirm and phospholipid corrections are not reported.
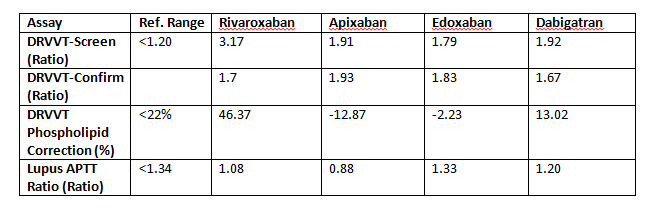
Some guidelines recommend mixing studies for Lupus Anticoagulant testing to determine whether a prolongation of dRVVT-screen/confirm is due to factor deficiency or genuinely due to the presence of a lupus. Both 1:1 (see table below) and 1:3 mixes with platelet poor plasma could not remove the effect of Rivaroxaban on both the dRVVT-screen and confirm assays resulting in weaker but still positive dRVVT phospholipid corrections.
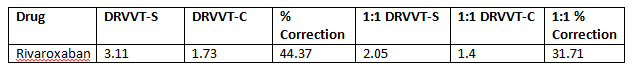
It is obviously not ethical to stop a patient’s anticoagulant for a prolonged period of time to enable thrombotic testing. It is therefore imperative that we have clear and up to date clinical details on the patients that we are testing to ensure that patients are not given an incorrect diagnosis. Below is the Lupus Anticoagulant results flow diagram in use in the Specialist Haemostasis Unit.