Emicizumab (ACE910) a new treatment for haemophilia patients has now recently been approved by the US Food and Drug Administration (FDA) under the trade name Hemlibra. The treatment is specifically developed for Haemophilia A patients with inhibitors, which have developed in response to their FVIII replacement treatment. Hemlibra is a recombinant, humanised, bispecific FIXa and FX directed antibody, designed to bring together factors IX and X to activate the coagulation cascade without FVIII. Hemlibra is used as a preventative treatment for bleeding episodes and can be administered subcutaneously as an injection. Here is a diagram showing the mode of action of emicizumab and how it mimics the role of FVIII in the coagulation cascade.
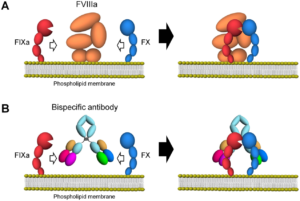
The use of the drug has been studied in the multicentre HAVEN clinical trials were its pharmokinetics, efficacy and safety were analysed. Participants were selected for the trial on the basis of being over 12 years of age, having congenital haemophilia A and having a history of a high titre FVIII inhibitor of over >5 Bethesda Units per mil of blood. Many patients will benefit from not having to have regular treatments and visits to a haemophilia centre. However, it is not quite understood how this drug will react with certain coagulation assays performed to previously monitor these patients. With the first patients already being treated with this drug in many Haemophilia Centres across the country it may require a new approach to laboratory testing in these patients.
References
Oldenburg, J et al . (2017). Emicizumab Prophylaxis in Hemophilia A with Inhibitors. The New England Journal of Medicine. 377 (9), 809-818.
Image taken from: http://www.huanqiuyixun.com/drugnews/5145.html