A full thrombophilia screen consists of Lupus Anticoagulant, Antithrombin, Protein C, Protein S and Activated Protein C (APC) Resistance assays. These assays were tested within one hour of sample collection for a baseline reading. They were then re-analysed at 8, 24, 48, and 72 hours post baseline (Figure 1).
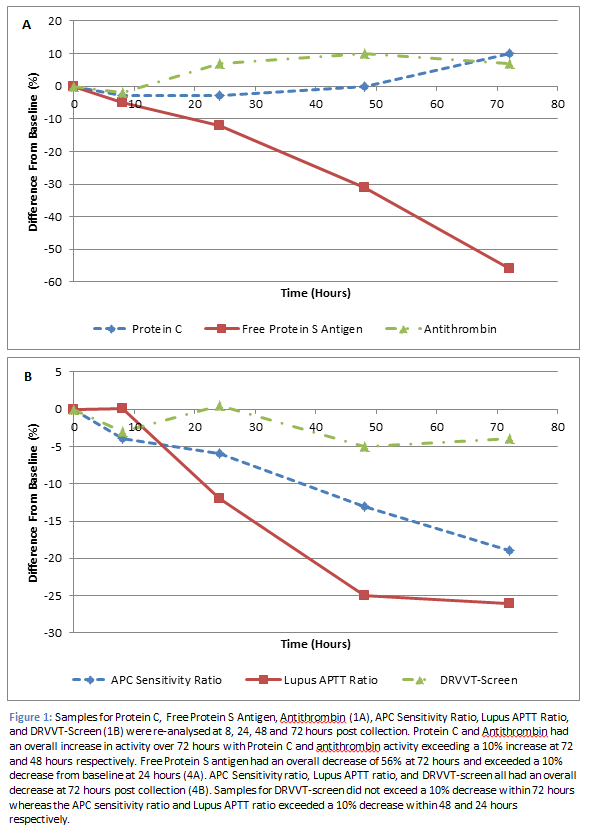
Lupus Anticoagulant (n=12) was measured via three assays: Dilute Russell Viper Venom Time (DRVVT) screen, APTT-SP, and APTT-DADE. The DRVVT screen produced significantly different results, compared to baseline, after 48 hours (p<0.01); however only a 5% decrease was observed within 72 hours. The APTT-SP and APTT-DADE are reported as a Lupus APTT ratio. This ratio produced significantly different results from 24 hours (p<0.01) and exceeded a 10% decrease from baseline at this time point (-11.8%). Antithrombin levels (n=8) increased from baseline reaching a significant increase (p=0.03) of 10% at 48 hours. Protein C levels (n=8) increased from baseline, producing a significant increase of 10% (p<0.01) at 72 hours. APC resistance (n=8) is tested using two assays, one which contains activated protein C (APC +) and one which does not (APC -), these results are then reported as a ratio of one another. APC ratio results significantly decreased from 8 hours (p=0.04), with a 13% decrease observed at 48 hours. Protein S levels (n=8) significantly decreased (p=0.03) within 8 hours of initial testing. However, the difference from baseline only exceeded 10% after 24 hours (-12%), and reached a reduction of 56% at 72 hours post baseline.
Protein C and Antithrombin requests are valid for 72 and 48 hours respectively post collection at which point results increased by 10%. The increase in Protein C activity was most likely due to contact activation which, in the order of 200-300 U/L of Kallikrein activity, will result in a 10-20% increase in Protein C levels [1]. A similar mechanism is likely to be apparent with the increase in Antithrombin levels, which was also measured via a chromogenic assay.
Samples for Lupus anticoagulant will be valid for 8 hours post collection. Although the DRVVT-Screen did not exceed a 10% difference from baseline within 72 hours, the Lupus APTT ratio reached an 11% decrease within 24 hours and a maximum decrease of 25% within 72 hours. Therefore, samples processed outside of their stability may result in false negatives being reported. Differences in these assays will attribute to differences in sensitivities to factor deficiencies. The DRVVT-Screen reagent is unaffected by factor deficiencies (except Factor X and II), as the dilute Russell Viper Venom directly activates Factor X whereas Lupus APTT assays, as the name suggests, are both APTT assays, which use reagents with different levels of phospholipid and have different sensitivities to intrinsic factor levels/deficiencies [2]. Therefore, their stability can presumably be attributed to the Factor VIII stability.
A similar mechanism is observed with APC sensitivity ratio. APC- results increase up to 3% over 72 hours, which can again be attributed to the decrease in Factor VIII. However, APC+ results decrease by 16% over 72 hours. This could potentially be due to release of platelet Factor V, which is resistant to complete inactivation by APC thus resulting in decreased APC+ results. This had an overall effect of decreasing the APC Sensitivity ratio by 13% at 48 hours and 19% at 72 hours. In an opposite fashion to the Lupus APTT ratio, APC sensitivity ratios performed outside of their stability could result in false positives (APC resistance detected) being reported.
Free Protein S antigen was the most unstable of the thrombotic assays with levels reaching a 56% reduction from baseline at 72 hours post collection. Protein S is present in two forms in plasma: free Protein S (40%), or linked to the complement C4b-binding protein (C4BP, 60%). Only free Protein S has functional co-factor activity. Free Protein S was measured using an immunoturbidometric assay whereby free Protein S in plasma is adsorbed onto C4BP coated latex particles resulting in agglutination with a second latex reagent, which is sensitized with a monoclonal antibody directed against human Protein S [3]. The decrease in free Protein S Antigen could be due to decreased ability of free Protein S to bind to the monoclonal antibody directed against human Protein S. Studies have shown that at very low concentrations, thrombin has the ability to cleave free Protein S at the N-terminal end, which is enhanced by a low calcium environment. Once free Protein S has been cleaved, it has a decreased affinity to calcium induced interaction with phospholipids [4]. The monoclonal antibody used in the free Protein S Antigen assay, is directed against a part of the antigen, which is at the opposite side to the C4BP-binding site. This would coincide with the proteolytic prone N-terminal region of Protein S.
References
- Instrumentation Laboratory – Protein C Activity. 2013; 0020300500
- Toulon P, Eloit Y, Smahi M, Sigaud C, Jambou D, Fischer F, Appert-Flory A. In Vitro Sensitivity of Different Activated Partial Thromboplastin Time Reagents to Mild Clotting Factor Deficiencies. International Journal of Laboratory Hematology. 2016; 38: 389-396
- Instrumentation Laboratory – Free Protein S Antigen. 2013; 002002700
- Dahlback B. Purification of Human Vitamin K-Dependent Protein S and its Limited Proteolysis by Thrombin. The Biochemical Journal. 1983; 209(3): 837-846